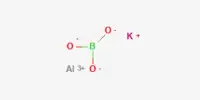
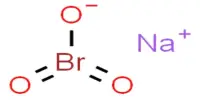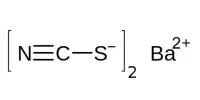Inertness of metal
Some trivalent metals like iron, chromium etc. has a special characteristic of reacting with dilute nitric acid easily. But they become inert in contact with highly concentrated or fluming nitric acid that means they lost their power of reaction. This state of inertness of metals is called the inertness of metal.
The reason of this inertness is the anti-reactive thin oxide layer created on the metal body produced by reaction of the acid with that metal. So the metal cannot come in contact with the acid any more. As far in case of iron, a layer of Fe3O4 is created on the iron body which ceases is ability to react further.
2HNO3 (conc.) = 2NO2 + H2O + [O]
3Fe + 4[O] = Fe3O4
This theory is confirmed by the fact that if the oxide film on the surface is removed by scratching or by heating it in a reducing atmosphere of H2 or CO or by dissolving it in iodine solution, iron again becomes active and begin, to give its usual reactions.
Fe3O4 + 4H2 = 3Fe + 4H2O