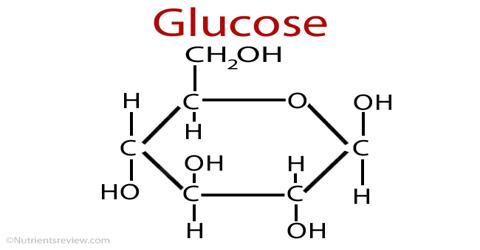
Glucose is a monosaccharide, which is another term for simple sugar. It is one of three monosaccharides that are used by the body, but it is the only one that can be used directly to produce ATP. ATP is used by the body for energy; in fact, ATP is the only molecule that can be used for energy. Another name of glucose is grape sugar or dextrose, (D-glucose). Unless you mean which elements compose the compound glucose: carbon, hydrogen, oxygen.
Chemical Properties – Glucose is the name of a particular chemical compound. The only constituent of glucose is glucose.
- Monosaccharides cannot be broken into simple CHO upon hydrolysis and they have 3 to 7 carbon atoms in their molecules.
- Oligosaccharides are made up of 2 to 10 units of monosaccharides or simple sugar.
- The general formula of polysaccharides is (C6H10O5)n; here. n = 100-300
- The monosaccharides which have aldehyde (—CHO) group in their structure is called aldose and the monosaccharides which contain ketone (>C=O) group in their structure is called ketose. Glucose, riboses are aldose: fructose is a ketose.
- Glucose contains four asymmetrical carbon atoms in its structure. If not you mean which subatomic particles create the atoms in a glucose molecule: protons, neutrons, electrons.
- It is not toxic and highly combustible (powdered glucose is extremely flammable).
- I can produce heat when it burns. And it flimsily dispersed particles can become volatile when they are exposed to air.
Physical properties – Physical characteristics find out whether the glucose molecule can be metabolized for energy and how it affects our health. When a few characteristics of a glucose molecule modify, it essentially becomes a dissimilar sugar.
- Can be solid or liquid,
- It has a sweet taste,
- Melting Point: 294.8˚F(146˚C),
- Density: 1.54 g/cm³,
- Weight: 180.16 g/mol,
- It has no odor,
- Soluble in water and acetic acid,
- All forms of glucose are colorless and are also clear.