Ferric hydroxide sol (hydrolysis)
THEORY: Ferric hydroxide forms a lyophobic sol with water which is the dispersion medium. It is prepared by the hydrolysis of ferric chloride with boiling distilled water as per the reaction:
FeCI3 (aq) + 3H2O → Fe(OH)3 + 3HCI (aq).
The HCI produced during the reaction tries to destabilize the sol and therefore should be removed from the sol by dialysis. A wine-red sol of ferric hydroxide is obtained.
Aim: To prepare a colloidal sol of ferric hydroxide.
Apparatus – Conical flask, beaker, a boiling-tube, glass-rod, funnel, round-bottom flask, iron stand with a clamp, wire-gauze, tripod-stand, burner, and a burette or a dropper.
Materials Required – 2% solution of ferric chloride (prepared by dissolving 2 g of pure FeCl3 in 100 ml distilled water) and distilled water.
Preparation
A freshly prepared nearly saturated solution of ferric chloride is first made. About 10 to 12 mL of the ferric chloride solution is then added dropwise to about 800 mL of boiling water. A few minutes interval is kept between, successive drops. A brilliant dark red sol is produced by the hydrolysis of ferric chloride forming ferric hydroxide and hydrochloric acid.
Ferric hydroxide forms a hydrophobic sol. Take 50 ml of distilled water in a beaker and heat it to about 100°C. Add the sol of FeCI3 to water with stirring. Hence it is prepared indirectly by the hydrolysis of ferric chloride with boiling water. The reaction takes place as follows:
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
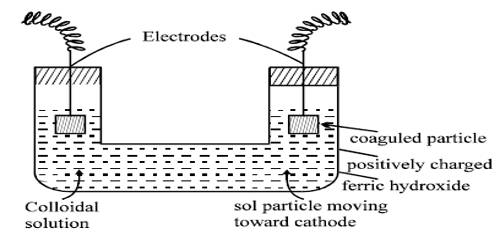
The ferric ions (Fe3+) produced from FeCl3 solution are absorbed on the surface of the particles of Fe(OH)3. As a result, the colloidal particles of Fe(OH)3 acquire a positive charge. Hence because of similar charges on the colloidal particles, they keep on repelling each other and cannot come together or aggregate together to form bigger particles. This prevents their coagulation and is responsible for the stability of the sol. However, the HCl formed simultaneously with Fe(OH)3, destabilizes the sol. Hence HCl, being an electrolyte is removed from the colloidal sol by dialysis.
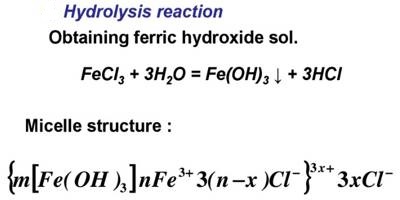
The sol needs immediate dialysis against hot water to remove the hydrochloric acid; otherwise, the sol would be coagulated. Many sols of heavy metal hydroxides can be prepared in this way.
Observation – A wine red sol is obtained
Inference – Sol of ferric hydroxide has been prepared
RESULT— Colloidal sol of ferric hydroxide has been prepared.
Precautions
- Since ferric hydroxide sol is affected by impurities, the apparatus required for the preparation of sol should be methodically cleaned by steaming-out process.
- Add ferric chloride solution drop-wise.
- Heating is continued till the desired sol is obtained.
- Hydrochloric acid formed as a result of hydrolysis of ferric chloride is removed by dialysis process otherwise it would destabilize the sol.
Application of Ferric Hydroxide –
- To abate water pollution, many governments are implementing stringent wastewater treatment policies.
- Various industries such as chemical, pharmaceutical, oil & gas, paper & pulp, and others, are using chemicals for sustainable treatment of water.
- These are becoming more popular for reducing concentration of toxic metals such as arsenic, copper, zinc, etc., in industrial water due to its specific chemical properties.