Factors Affecting the Rates of Reaction
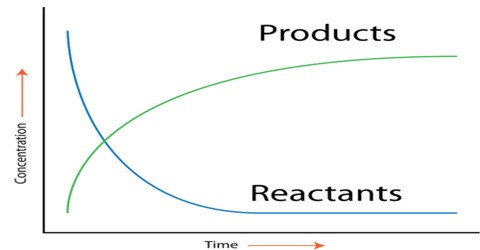
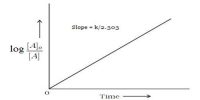
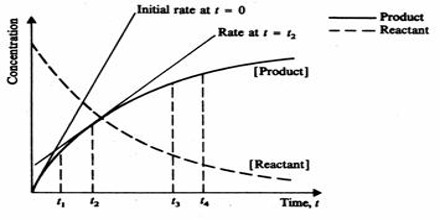
The rate of a reaction (rate) is the amount of reactant used up or the amount of product formed in unit time. In the application in chemistry, the amount is expressed in moles, or more usually in mol L-1. The rate of a chemical reaction is the decrease in concentration (in mol L-1) of the reactant or an increase in the concentration of the product in unit time. The unit of the rate is usually expressed as mol L-1 s-1 (or min-1, h-1 etc. depending on the ‘fastness’ or ‘slowness’ of the reaction).
Experiments show that the rates of reactions are affected by several factors variables. These are:
(a) A concentration of the reactant
(b) Pressure (in the case of gases)
(c) Temperature of reaction
(d) Catalyst
(e) A surface area of solid reactant or catalyst.
And, in some cases, light,
The effect of concentration, pressure and surface area on the rate of a reaction can be simply explained if one recognizes that in order for a reaction to take place reactant particles must collide with each other. More the number of collisions in unit volume in unit time higher is the rate of a reactor The effect of temperature is two-fold:
- an increase of temperature increases the kinetic energy of the reactant species bringing about an increase in the frequency of collisions,
- at higher temperature more reactant species have the required energy of activation.
Both of these factors increase the rate when a temperature is increased. A catalyst provides an alternative path with the lower activation energy for the reaction so that in the presence of a catalyst more reactant species have the necessary activation energy and the rate increases. Well, known examples of the effect of light on the rate of a reaction are photosynthesis and the reaction of hydrogen and chlorine, which do not take place in the absence of light.