Extraction of Transition Elements:
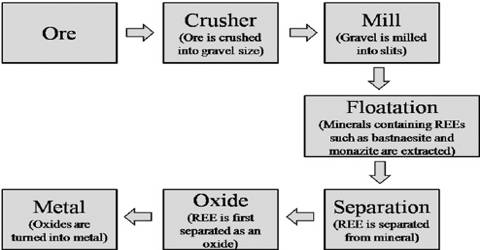
Principle→ Transition elements are mainly found as oxide & sulphide ores in nature. The method used to extract metals from the ore in which they are found depends on their reactivity. For example, reactive metals such as aluminium are extracted by electrolysis, while a less-reactive metal such as iron may be extracted by reduction with carbon or carbon monoxide. The main steps of extraction are as follows:
(i) Concentration / Condensation of ore: It is done by 2 ways:
- Generally slog is removed from oxide/ carbon ores by washing with water.
- If sulphide compound is present in ore, slag is removed by oil-froth flotation process.
(ii) Roasting: done in a current of air.
(iii) Smelting: by carbon-reduction or self-midation methods.
Reduction of metallic oxide (in case of TiO2 to Ti) by Na & Mg → Metallic reduction. Reduction of metallic oxide (in case of V2O5 to V and Mn3O4 to Mn) by Al → Thermit Reduction.