Extraction of Titanium
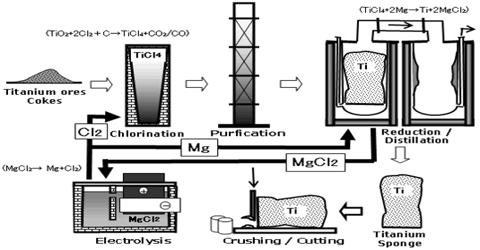
According to abundance in earth’s crust, it is in ninth position (0.6%). About 7-10% Titanium dioxide (TiO2) is found in the stone of Moon’s surface; which was collected by the space craft Apollo- 11. Titanium is extracted from its ore, rutile – TiO2. It is first converted into titanium chloride, which is then reduced to titanium using either magnesium or sodium. The ore rutile (impure titanium oxide) is heated with chlorine and coke at a temperature of about 1000°C.
It is extracted from its ore by 03 processes→
(ii) Production of Titanium tetrachloride (TiCL4) from ore: It is done by chlorine process. Coke carbon is mixed with smashed limonite & heated in 3600 C. The Chlorine (Cl2) gas is passed through the mixture. Ferric Chloride (FeCl3) & Titanium tetrachloride (TiCl4) are produced. The reaction is-
2FeTiO3 – 3C + 7Cl2 →3600C→ 2FeCl3 + 2TiCl4 + 3CO3
TiO3 + 2C + 2Cl2 →3600C→ TiCl4 + 2CO2
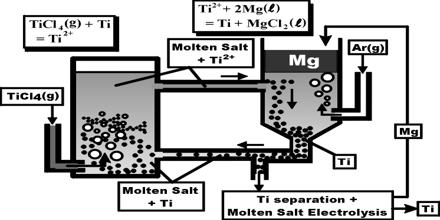
(ii) Production of Ti from TiCl4: It is done by Croll’s process
(iii) Purification of Ti: by Iodine.
Titanium alloys are light, resistant to corrosion, and they remain strong at high temperatures. These properties make them useful in many applications, including aircraft parts and artificial joints.
Uses of Titanium:
- Ti is lighter than steel & it isn’t easily decayed. It is also stronger & harder than Al.
- It is more heat-stable than steel, so it is used in making capsule of space-crafts.
- It is used in nuclear plants, pace-makers, chemical plants & hip pint replacement.
- Micro-instruments of radio & televisions, different white dyes are made from Ti.
- It is also used in different architectures; e.g. historical monuments, sculptures etc.