The method used to extract copper from its ores depends on the nature of the ore. Sulphide ores such as chalcopyrite are converted to copper by a dissimilar method from silicate, carbonate or sulphate ores.
The principal ore of copper is copper pyrite and copper is extracted from this ore. Extraction of copper from copper pyrites involves the following steps –
Crushing and concentration
The ore is crushed and then concentrated by froth floatation process. The ore obtained from mines are broken down into little piece by jaw crusher and then pulverized. The ore being sulphide ore is concentrated by froth floatation procedure. Ore forms froth with pine oil and come to the surface and are skimmed off though impurities are left in water.
Roasting
The concentrated ore is heated strongly in the reverberatory furnace, in excess of air. The concentrated ore is heated in an excess supply of air on the hearth of a reverberatory furnace below its melting point. During roasting,
- Moisture is removed.
- The volatile impurities are removed.
Sulphur, phosphorus, arsenic, and antimony which are present as an impurity are removed as volatile oxides.
S + O2 → SO2
P4 + 5O2 → 2P2O5
4As + 3O2 → 2As2O3
The copper pyrite is partly converted into sulphides of copper and iron.
2CuFeS2 + O2 → Cu2S + 2FeS + SO2
2FeS + 3O2 → 2FeO + 2SO2
The Process in summary:
The concentrated ore is heated stoutly with silicon dioxide (silica) and air or oxygen in a furnace or series of furnaces.
- The copper (II) ions in the chalcopyrite are condensed to copper (I) sulfide (which is condensed more too copper metal in the last stage).
- The iron in the chalcopyrite ends up transformed into an iron (II) silicate slag which is removed.
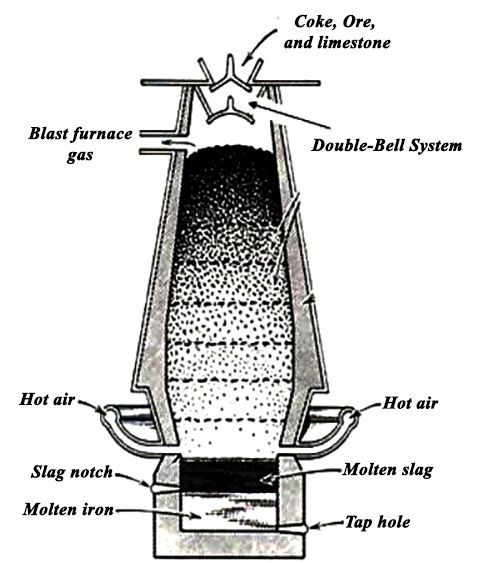
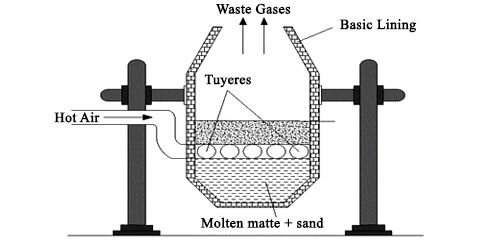
Smelting
The roasted ore is mixed with powdered coke and sand and is heated in a blast furnace. It is made of steel plates lined inside with fire clay bricks. Hot air at 800°C is introduced from the tuyers near the base of the furnace. As a result, the following changes occur.
- 2FeS + 3O2 → 2FeO + 2SO2
- FeO + SiO2 → FeSiO3 (fusible slag)
- 2Cu2S + 3O2 → 2Cu2O + 2SO2
- Cu2O + FeS → Cu2S + FeO
- FeO + SiO2 → FeSiO3 (fusible slag)
As a result of smelting, two separate molten layers are formed at the bottom of the furnace. The upper layer consists of slag and is removed as a waste while the lower layer is called matte. It chiefly consists of cuprous sulphide and some unchanged ferrous sulphide.
Refining: Blister copper contains about 2% of impurities and it is purified by electrolytic refining.
Physical Properties:
- It is a transition metal having a characteristic red color,
- It has high melting point 10830c and bpt 2320c,
- It is highly malleable and has high electrical and thermal conductivity,
- It has a specific gravity 8.93.
Uses of Copper:-
- It is in making electrical cables.
- It is used in making coins.
- It is Rold Gold, constantan, bell metal, etc.
- It is used in making utensils.
- It is used in making scientific equipment like calorimeter, boilers, etc.