Brief concepts of Werner’s theory of coordination compounds
Alfred Werner studied the structure of coordination complexes and put forward his ideas in the year 1893 which were known as ‘Werner’s coordination theory.
Postulates of Werner’s theory
The important postulates of Werner’s theory are:
(i) The fundamental metal or the metal atoms in harmonization compounds show two types of valency. In coordination compounds, the central metal or metal atoms demonstrate two types of valency. They are the primary and secondary valency.
Every metal atom has two types of valencies
- Primary valency or ionizable valency
- Secondary valency or non-ionizable valency
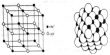
(ii) The primary valency corresponds to the oxidation state of the metal ion. The primary valency of the metal ion is always satisfied by negative ions. The metal atom tends to persuade its both primary and secondary vacancies. Primary valency is contented by negative ion whereas secondary valances are contented by negative ion or by impartial molecules. If the metal ion has four secondary valences, these arrange in either tetrahedral or square planar arrangement around the central metal ion.
(iii) Secondary valency corresponds to the coordination number of the metal ion or atom. The secondary valencies may be satisfied by either negative ions or neutral molecules. The metal atom works towards satisfying both its primary and secondary valencies. An unenthusiastic ion satisfies the primary valency. Every metal atom has a permanent number of secondary valencies, For Example, It has predetermined harmonize number. On the other hand, a negative ion or impartial molecules convince secondary valencies.
(iv) The molecules or ion that satisfy secondary valencies is called ligands. The ligands which satisfy secondary valencies must project in definite directions in space. So the secondary valencies are directional in nature whereas the primary valencies are non-directional in nature. The ligands have unshared pair of electrons. These unshared pair of electrons are donated to central metal ion or atom in a compound. Such compounds are called coordination compounds.
(v) The primary valency corresponds to oxidation condition and the secondary valency corresponds to harmonize number. The primary valency relates to the oxidation state and the secondary valency relates to the synchronize number. The number of secondary valences is permanent for each metal atom. It means that the harmonization number is predetermined.
(vi) The secondary valences point towards a permanent situation in space. The secondary valances are always directed towards a permanent position in space and this cause specific geometry of the synchronize compound. This is the cause behind the exact geometry of the synchronize compound.