Formation and structure of ethylene molecule:
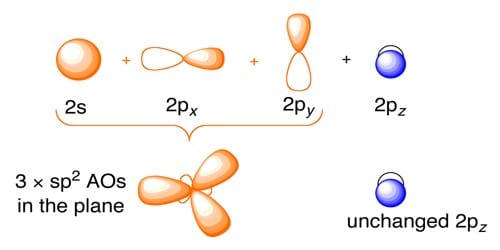
In ethylene and in other organic compounds having C = C bond, 2s and two 2p orbitals of carbon atom undergo sp2 hybridization.
At normal state the electronic configuration of carbon atom C(6) = ls2 2s2 2px1 2py1 2pz0
At excited state the electronic configuration is C*(6) = ls2 2s1 2px1 2py1 2pz1
After sp2 hybridization the electronic configuration is C*(6) = 1s2 ψ11 ψ21 ψ31 pz1
Where ψ1, ψ2, ψ3 are sp2 hybrid orbitals. Meanwhile, out of 2s, 2px, 2py, and 2pz orbitals in carbon, only 2px, 2py, and 2s take part in hybridization. Then are hybrid orbital of one carbon atom overlaps with one hybrid orbital of another carbon atom and thus form a C-C sigma (σ) bond. Two other hybrid orbitals of each carbon atom overlap with 1s orbitals of two hydrogen atoms and thus form two C-H bonds with each carbon atom. One 2pz orbital remains unchanged. This leads to the formation of three sp2 hybridized orbitals. Thus the main structure of ethylene is built. Due to sp2 hybridization, the bond angles are ethylene arc about 120° and all the atoms are on a plane. One carbon atom overlaps the sp2 orbital of another carbon atom to form an sp2 – sp2 sigma bond.
Then the 2pz orbital on each carbon atom remains with one electron each. The two sp2 hybrid orbitals get overlapped by two hydrogen atoms containing unpaired electrons. They overlap with each other sidewise to form one C-C pi-bond. A pi bond is formed by the unhybridized 2pz orbitals of each carbon atom. There is a formation of a sigma bond and a pi bond between two carbon atoms.
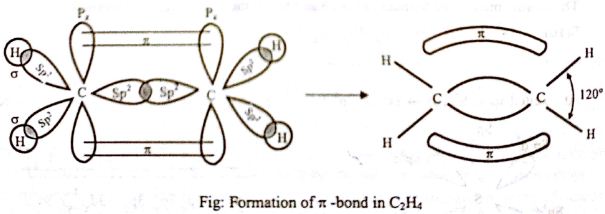
When we look at the molecules of C2H4 it has 2 CH molecules and 4 H molecules. The carbon atom consists of 6 electrons and hydrogen has one electron. During the formation of CH2 = CH2, the electronic configuration of carbon in its ground state (1s2 2s2 2p1 2p1) will change to an excited state and change to 1s2 2s1 2px12py1 2pz1.
Important Points to Remember
- Ethene (C2H4) has a double bond between the carbons. In the ethene molecule, the carbon atoms are sp2 hybridized. One unpaired electron in the p orbital remains unchanged. The two carbon atoms form a sigma bond in the molecule by overlapping two sp2 orbitals.
- In ethylene, each carbon combines with three other atoms rather than four. The three hybridized orbitals explain the three-sigma bonds that each carbon forms.
- The pi bond between the carbon atoms perpendicular to the molecular plane is formed by 2p–2p overlap.