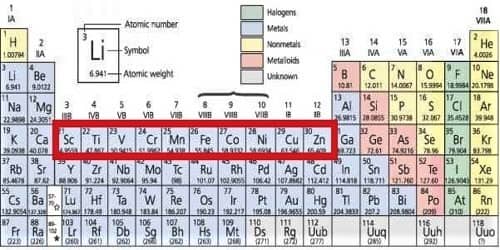
All the elements of the d block are transition metals. Transition elements are those elements that have partly or moderately filled d orbital in their ground state or the most constant oxidation state. The cations of d-block elements have a strong tendency to form complexes with certain molecules (e.g. CO, NO, NH3….etc) or ions (e.g. F-, Cl-, CN- ….etc) called ligands. Their tendency to form complexes is due to two reasons.
- Small size and high positive charge density. Transition metals have a little size and high charge density constitutes a strong electrostatic force of magnetism with the ligands and does constancy of the complex increases.
- Presence of vacant (n-1)d orbitals which are of appropriate energy to accept lone pair and unshared pair of electrons from the ligands for bonding with them. Transition elements having a huge number of empty orbitals in which it can contain the lone pair of electrons provided by the ligands, therefore, can play the role of Central metal ion or central metal atom.
Examples of some complex compounds are,
[Cu(NH3)4]2+, [Ag(NH3)2]+, [Fe(CN)6]4-,….etc.
They are of proper energy to believe lone pair and unshared pair of electrons from the ligands. Hence conversion elements form complexes simply. Most subsist in numerous oxidation states and form many multifaceted ions and colored compounds. The partly filled subshells of d-block elements incorporate (n-1) d subshell. The leftmost members in a phase in the d block are active metals, but those to the right in the stage are fewer active. All the d-block elements hold a comparable number of electrons in their outmost shell. Therefore, they acquire comparable chemical properties.