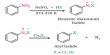
Fluorine differs from the rest of the elements of its family due to (i) its small size (ii) highest electronegativity, (iii) low bond dissociation energy, and (iv) absence of d-orbitals in the valence shell.
Anomalous Nature of Fluorine
- Fluorine is the most reactive element among halogen. This is due to the minimum value of the F–F bond dissociation energy. It is most reactive of all the halogens due to lower value of F – F bond dissociation energy (F2 = 158, Cl2 = 243, bromine = 192 and Iodine = 151 kJ mol-1) .
- Fluorine decomposes cold dilute alkalies liberating OF2 and with conc. alkali, O2 is liberated. Under similar conditions, the other halogens will give rise to the hypohalites and halates respectively.
- Being the most electronegative element, it shows only an oxidation state of -1 and does not show positive oxidation states due to the absence of d-orbitals in its valence shell. Other halogens show positive oxidation states of +1, +3, +5, and +7.
- It has the greatest affinity for hydrogen, forming HF which is associated due to hydrogen bonding. Hydrofluoric acid is a weak acid whereas the other hydrohalic acids are strong acids.
…… H– F…… H– F….. H– F.
- It differs markedly from the other halogens in that it can form two types of salts with metals. NaF and NaHF2.
- The salts of HF differ from the corresponding salts of other hydracids. AgF is soluble in water while the other AgX is insoluble. Fluorides have the maximum ionic character. For example, .A1F3 is ionic while other halides of Al are covalent.
- Due to small atomic size and high electronegativity of F, HF undergoes strong H-bonding while other halogen acids do not. Being strongly electronegative it can have only a negative oxidation state while the other halogens can have negative as well as positive oxidation state.
- HF attacks glass while others do not. HF is a liquid (boiling point 292.5K), while other halogen acids are gases at room temperature (boiling point of HCI = 189K, HBr = 206K, HI = 238K). HF is the weakest of all the halogen acids due to the high strength of H – F bond.
- Fluorine, because of the absence of d-orbitals in its valence shell does not form any polyhalides. Fluorine is smallest in size and d-orbitals are not available in the valence shell of fluorine. hence it has no tendency to form polyhalite in whereas other halogen form polyhalite ions like – C13–, Br3–, I3– . Thus we have Ι3–, Br3–, Cl3– ions but no F3– ion.
- Fluorine has the highest electronegativity other halogens. Fluorine forms strong hydrogen bonding in its hydrides, unlike other halogens, because the H-F bond is highly polar in nature. Hence HF is a liquid while other hydrogen halides are gauses at room temperature.
Fluorine exhibits anomalous behavior as compared to other halogen atoms in the group. The reasons for the anomalous behavior of fluorine are as follows.
(i) The smallest size of fluorine,
(ii) The highest electronegativity
(iii) Low bond dissociation enthalpy of F- F. bond
(iv) Non-availbility of d-orbitals in the valence shell