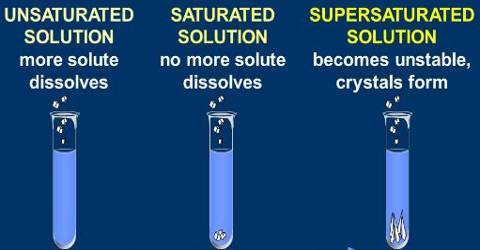
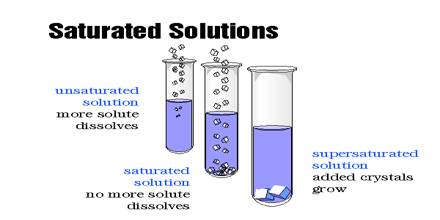
A saturated solution is that which is dissolved as much solute as it is capable of dissolving. By heating the solution a saturated solution can be altered into an unsaturated solution. Without adding any solvent it can be changed into an unsaturated solution. An unsaturated solution contains less than the maximum amount of solute that can be dissolved at that temperature. Example: on heating a saturated solution of sugar at high temperature, it starts dissolving.
If more solute is added and it does not dissolve, then the original solution was saturated. If the added solute dissolves, then the original solution was unsaturated. A saturated solution can dissolve no more of the solute. Hence, the size of the crystal will remain the same.
Experiment –
Task: To make saturated and unsaturated solutions. In order to find out if a solution is saturated or unsaturated, we need to put a crystal or soluble solute in the solution.
Required accessories: One beaker, measuring flask, stirrer, salt, and water.
Procedure: Clean the beaker by its washing well. Use the measuring flask to take 100 milliliters of water in the beaker. Now keep adding salt in little amounts to the water and stir. In this way continue to add salt and stir until the added salt does not dissolve anymore despite rigorous stirring.
Analysis:
A saturated solution is when the solute can dissolve in the solvent. For example, if you have a bottle of water and you pour lemonade crystals into the water, and it dissolves, the solution is saturated. An unsaturated solution is when the solute cannot dissolve into the solvent. For example, if you have a glass of water, and you pour something like vegetable oil into it, it will not dissolve. So that makes the solution unsaturated
In course of adding salt in little chunks, why, at a certain stage, the salt does not dissolve despite constant stirring? The cause is, in the process of adding salt at regular intervals, the solution has become saturated. This is a state when the solvent (water) is unable to dissolve any more solute (salt). So, at a certain temperature if the maximum amount of solute that can be dissolved by a certain amount of solvent is dissolved by the solvent, then the solution obtained is called a saturated solution.
On the other hand, is a solution, if the amount of solute dissolved is less than the maximum amount of solute the solvent can dissolve, that solution will be an unsaturated solution.
In the above events, the solution obtained in all the phases before the last chunk of added salt that remained undisclosed are examples of unsaturated solution. In a saturated solution, a little amount of the solute will not be dissolved in spite of much stirring. On the other hand, in an unsaturated solution, the added solute will continue to dissolve due to stirring until the solution converts into a saturated solution.
Now, in the case of a supersaturated solution, there is an excess solute in the solution so, it wants to leave its excess solute, due to which added crystal or solute will become bigger in size than before.
Some of the examples of a structured solution are –
- Carbonated water is saturated with carbon; hence it gives off carbon through bubbles.
- Adding sugar to water until it no longer dissolves creates a saturated solution.
- Continuing to dissolve salt in water until it will no longer dissolve creates a saturated solution.