Enthalpy of Ionization or Ionization energy
When an electron is removed from an atom a cation is formed. In this process energy will be absorbed. From an atom the electrons can be removed one by one and each step will be accompanied by absorption of energy. Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation. The successive ionization energies are called the first ionization energy, second ionization energy etc. For comparing ionization energies of different elements the following definition is given:
The first ionization energy of an element is the energy required to remove one electron from each of one mole of gaseous atoms to form one mole of unipositively charged gaseous cations.
The equation for the process is;
X (g) → X+ (g) + e; ∆H0 = First ionization energy
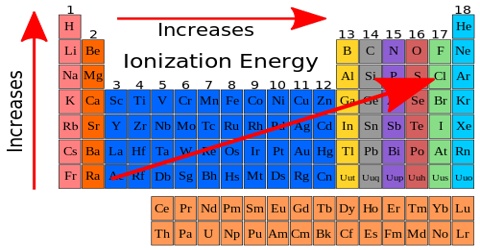
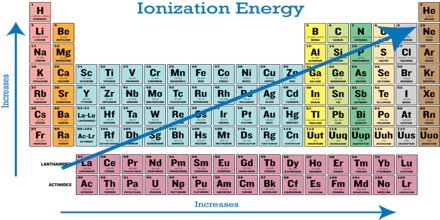
For elements from left to right in a Period (in the Periodic Table), say from Na to Ar in the third Period, there is an increasing trend at the first ionization energy as the attraction for the outer electron by the nucleus increases. The ionization energy of an atom is the amount of energy needed to remove an electron. Since potassium is more reactive than sodium, it takes less energy to remove an electron. Also, potassium will have lower ionization energy since it is in a higher energy level than sodium. Down a Group the first ionization energy decreases as the outer electrons get farther away from the nucleus of the atom and their Ail 1 attraction by the nucleus decreases due to increasing shielding. The successive ionization energies for a particular element, however, increase as the electrons are removed one by one.