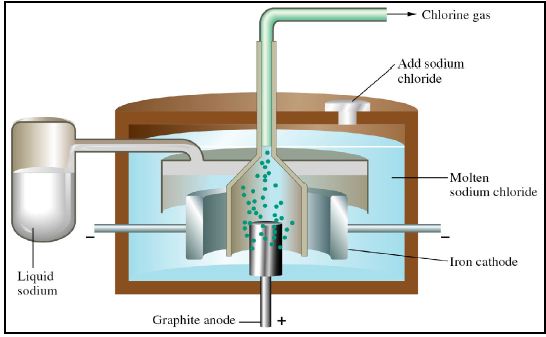
A Downs cell is a commercial electro- chemical cell used to obtain sodium metal by electrolysis of molten NaCI. A number of other reactive metals are obtained by the electrolysis of a molten salts. Many important substances, such as aluminium metal and chlorine gas are produced commercially by electrolysis.
Aqueous electrolysis
Electrolysis of molten ionic compounds will yield the elements from the salt, however, electrolysis of aqueous solutions of salts often result in the oxidation and/or reduction of the water instead, yielding H2 and O2. For example, the electrolysis of concentrated NaC1 solution yields C12 at the anode, but H2 rather than Na at the cathode.
In an electrolytic cell, the anode has a positive charge. In a voltaic cell, the cathode has a positive charge. In a voltaic cell, the anode has a negative charge.
Cations (ions of positive charge) will be attracted to the negative cathode. Anions (ions of negative charge) will be attracted to the positive anode. For example, during the electrolysis of molten NaCI:
– Na+ ions are attracted to the cathode, where they will be reduced to Na.
– Cl– ions will be attracted to the anode, where they will be oxidised to Cl2.