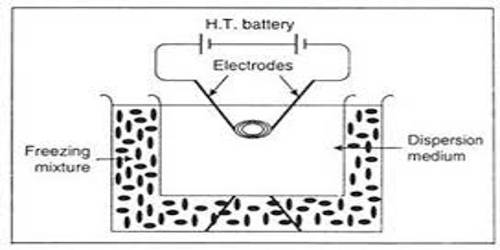
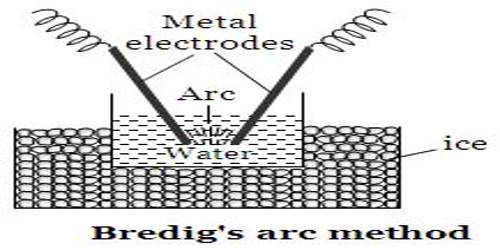
Electrical disintegration: Bredig was the originator of disintegrating metals into sols by means of an electric arc. Two metallic electrodes dipped in water, as shown in Figure are connected to a D.C. source. A rheostat and an ammeter are connected in series for controlling current. On passing the current an arc is produced near the ends of the electrodes and a metal sol results.
A small quantity of sodium hydroxide is added to water. The sol thus produced consists of metal in case of noble metals like gold, platinum, but the sol is mainly oxide or hydrous oxide, in case of metals like zinc, aluminum, chromium, iron etc. Svedberg improved the technique by using an A.C. source and working at high current densities. He succeeded in preparing not only hydrosols but also organosols. It is thought that under the influence of the high temperature of the arc the metal is first vaporized and the colloidal particles formed later by condensation.
A special class of colloid can be prepared by simply heating the substance with the dispersion medium. These are molecular colloids 1.e., substances of high molecular mass but the molecules attain colloidal dimension due to their largeness Solutions of high polymers like proteins, cellulose etc. fall under this category.
The two general methods given for preparation of colloids are not to be taken as the last word. The preparation of each different colloid needs special conditions and each system must be separately studied. What is good for one system need not necessarily be good for the other. Each individual colloid needs individual attention and quite often the control of conditions is to be rigid. Even then for some unknown reasons, one attempt fails and the other succeeds. It is not too much to say that preparation of colloids is as much science as it is art.