Direction of chemical change
It is possible to calculate free energy change of a reaction and decide whether the reaction will take place spontaneously under the given conditions or not. The free energy change (∆G) in a reaction may be obtained from calorimetric or other means of finding ∆H and ∆S. The relation ∆G = ∆H – T ∆S, is used to determine the sign and magnitude of ∆G. It is clear that to make ∆G negative, ∆H should be negative (exothermic) and ∆S positive. For endothermic reactions (positive ∆H), ∆G can be negative only if ∆S is positive and T∆S is larger in magnitude than ∆H. A reaction with a negative ∆S value may also take place spontaneously if the reaction is highly exothermic (∆H negative) and ∆H is larger in magnitude than T∆S. Thus, the sign of ∆G can predict the direction and the feasibility of a process.
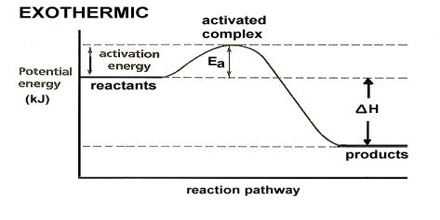
A negative value of ∆G for a given process does not necessarily mean that the process will take place. These calculations do not say how long it would take for the process to reach equilibrium. In fact, thermodynamics has nothing to say about the speeds of reactions. It can only predict the direction and the possibility of the process taking place.
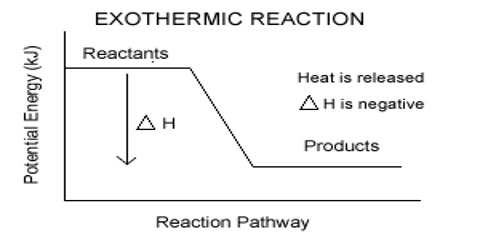
The rate and direction of a chemical reaction depends on the free energy, entropy, and concentration of the reactants and products as well as the temperature and pH of the system. Chemical reactions progress in the direction of high to low energy. We can estimate the direction of the chemical reaction, as well as the equilibrium concentrations of reactant and product, by examining the energy of the reactants and products.