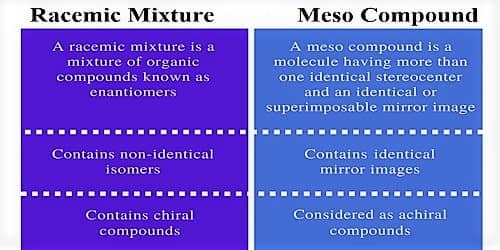
A racemic mixture is a combination of organic compounds known as enantiomers. It is optically dormant due to the presence of equal amounts of non-super imposable mirror images. A meso compound is a molecule having more than one identical stereocenters and an identical or superimposable mirror image. A meso compound contains an identical mirror image. The key dissimilarity between a racemic mixture and a meso compound is that a racemic mixture contains non-identical isomers whereas a meso compound contains an identical isomer.
Differences between the racemic mixture and meso compound –
Racemic mixture – A mixture that contains equal quantities of enantiomers is called a racemic mixture.
- Definition: A racemic mixture is a mixture of organic compounds known as enantiomers.
- The equimolar mixture of two enantiomers is called racemic mixture e.g (+) and (-) lactic acid forms (±) – lactic acid as a racemic mixture.
- Composition: A racemic mixture contains non-identical isomers.
- A racemic mixture is optically inactive due to external compensation.
- It can be separated into two enantiomers which are optically active.
- Chirality: A racemic mixture contains chiral compounds.
- The racemic mixture may be formed from one or more than two asymmetric centers. Its optical inactivity is due to external compensation. The separation of a racemic mixture into D & L form is called resolution.
- A racemic mixture is a 50:50 combination of a pair of enantiomers with the mixture having zero optical rotation.
- Racemic compounds are the mixture of dl-compounds which chiral and optically active.
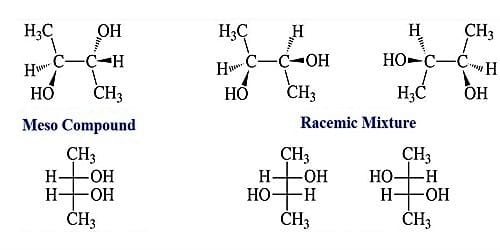
Meso compound
- Definition: A meso compound is a molecule having more than one identical stereocenter and an identical or superimposable mirror image.
- A meso compound has at least two identical asymmetric centers with a plane of symmetry e.g. meso tartaric acid.
- Composition: A meso compound has identical mirror images.
- Meso compound is optically inactive due to internal compensation.
- Meso compound is a single compound which cannot be separated into an optically active compound by the resolution process.
- Chirality: Meso compounds are considered as achiral compounds.
- Meso compounds should have at least two identical chiral centers. The meso compound is achiral (optically inactive) even though it has two stereocentres. The Meso compounds have two features in common: (a) a plane of symmetry and (b) two stereocentres with opposite stereodescriptors
- Meso-form is a compound with two or more chiral centers and a plane of symmetry. It is optically inactive due to internal compensation or self-canceling stereocentres of opposite configuration.
- Meso compounds are those chiral compounds which are optically inactive. A meso compound occurs when a molecule with two chiral centers possess a plane of symmetry. Although the molecule has two chiral centers yet overall the molecule is achiral.