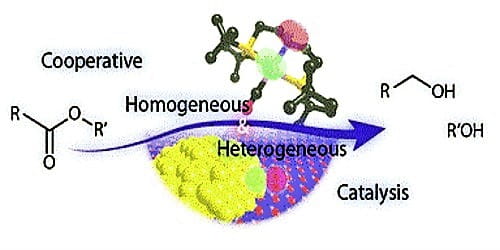
Homogeneous catalysis takes place when the catalyst and the other reactants are all dissolved in the same solution. Heterogeneous catalysis typically involves the use of a catalyst that is insoluble, or perhaps only weakly soluble, in the solution in which the reaction takes place. Thus, in heterogeneous catalysis, the catalyst and solution may form a suspension, or the catalyst may simply be a solid that is placed in the solution.
Differences between Homogeneous Catalysis and Heterogeneous Catalysis are mentioned below:
Homogeneous catalysis
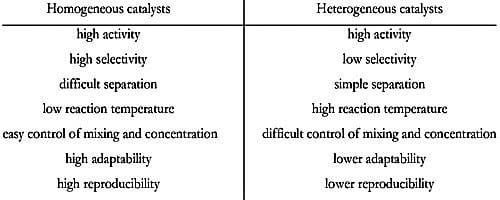
- Homogeneous catalysts are catalytic compounds that are in a similar phase as the substances which are going into the reaction phase.
- These types of catalysts usually are common in either the liquid phase or gas phase
- Operative temperature for homogeneous catalysis is generally low except only when under high pressure.
- The recovery of homogeneous catalysts is complicated and exclusive.
- The partition of homogeneous catalyst from the reaction mixture is complex.
- The heat transfer in homogeneous catalysis is very high as all the molecules of reactants and catalysts are in the same phase.
- Recycling methods are not very cost effective as it’s a long drawn process and as it’s a difficult treatment method for spent catalysts
- Modification of homogenous catalysts is very easy as it depends upon the tuning of electronic and steric properties on metal
- The recycling of homogeneous catalysts is not easy. The reaction mechanism is easier to find as varied techniques are available.
- The dynamic site of homogeneous catalysts is well-defined and has good selectivity.
- The thermal constancy of homogeneous catalysts is poor.
Homogeneous catalysts work better in low-temperature conditions (less than 2500C). In which the reactants and catalyst are in a similar phase. Example: 2SO2(g) + O2(g) —– No(g) → 2SO3(g).
Heterogeneous catalysis
- Heterogeneous catalysts are catalytic compounds that are in a contradictory phase from that of the phase of the reaction combination.
- Heterogeneous catalysis is found in the liquid phase, gas phase, and solid phase.
- Operative temperature for heterogeneous catalysis is harsh as compared to the homogeneous process.
- The recovery of heterogeneous catalysts is simple and inexpensive.
- The severance of heterogeneous catalyst from the reaction combination is simple.
- The heat transfer is relatively low as compared to homogeneous catalysis as the reactant molecules and catalysts are in a different phase.
- These catalysts although require reactivating treatment process but still quite cost effective.
- The modification of heterogeneous catalysts is relatively difficult as the controlling methods of particle size, as well as the active size at a molecular level, is really difficult.
- The recycling of heterogeneous catalysts is simple. The reaction mechanism is difficult to fins as the techniques utilized as the product is scrutinized and not the catalysts.
- The dynamic site of heterogeneous catalysts is not well-defined and has deprived selectivity.
- The thermal constancy of heterogeneous catalysts is superior. The severance of the catalyst from the reaction combination is simple.
Heterogeneous catalysts work enhanced in high-temperature conditions (around 250 to 5000C). In which the reactants and catalyst phases differ from each other. Example: 2SO2(g) + O2(g) —– pt(s) → 2SO3(g).