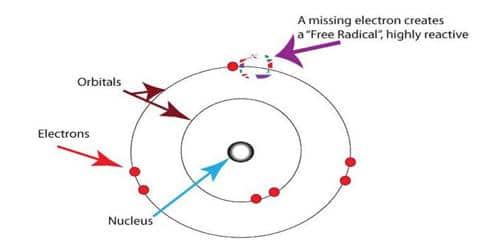
The key difference between free radicals and ion is that the free radicals have one or more unpaired electrons, but ions have paired electrons.
Differences between free radical and ionic reactions –
Free radical reactions
- The reactions proceed through free radicals produced by hemolytic fission of covalent bonds.
- They are catalyzed by light, peroxide, and high temperature.
- These reactions occur in non-polar solvents or in a gaseous state.
- They are usually chain reaction. Chain growth termination occurs via combination or disproportionation which competes with chain growth and chain transfer reactions.
- They are hindered by free radicals. Free-radical polymerizations require potent initiators (and co-initiators). The rate constant for initiator dissociation is much smaller than that for propagation.
- A radical has one or more unpaired electrons. E.g., Cl
- Initiation and propagation are strongly affected by the reaction temperature. An increase in temperature almost always increases the rate of chain propagation but it also increases chain transfer and thus lowering the molecular weight.
- The degree of polymerization depends on the velocity constants of radical formation, chain growth, chain transfer, and chain termination.
A radical polymerization begins when an initiator is split up in two radicals. During propagation, the monomer adds consecutively to the radical which becomes a growing polymer chain with a radical active center. At termination, the growing chain is combined with another radical, for example, another growing chain, or a chain transfer agent.
Polar or ionic reactions
- The reactions proceed through ions produced by heterolytic fission of covalent bonds.
- These reactions are catalyzed by acid or base.
- They occur in polar solvents.
- They have not chained reaction. There is no termination step unless contaminations such as water or alcohol are present or deliberately added to terminate the reactions.
- They are not influenced by free radicals. The initiation of anionic polymerization has typically a much smaller activation energy than the corresponding free-radical polymerization.
- An ion (cation or anion) typically has all electrons paired. E.g., Cl-
- Anionic polymerization reactions are rather insensitive to temperature and can be carried out at very low temperatures but are only suitable for monomers with strong electron-withdrawing groups.
- Anionic polymerization reactions yield more regular polymers with less branching, more controlled tacticity, and narrow molecular weight distribution.
Ionic polymerization also has these steps, but this time being initiated by an ion/counterion which becomes the active center and grows through nucleophilic monomer additions, and the termination is where the ion is neutralized or stabilized.