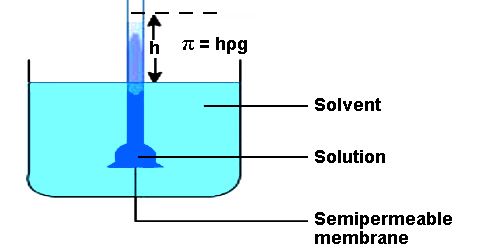
Osmotic pressure might be defined as the surplus pressure which must be applied to a solution in order to prevent the flow of solvent into the solution through the semi-permeable membrane.
Determination of Osmotic Pressure by Berkeley and Hartley’s method
Abbe Nollet (1748) was the first to observe the phenomenon of osmosis and made measurements of osmotic pressure. His measurements with a pig’s bladder as the semi-permeable membrane gave only semi-quantitative results as the pig’s bladder is not a good semi-permeable membrane.
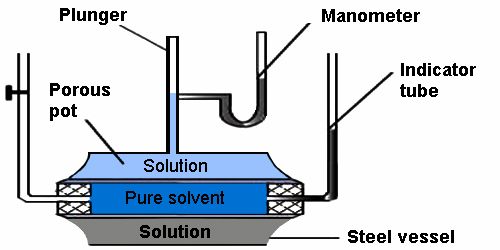
Fig: Osmotic Pressure by Berkeley and Hartley’s method
Berkeley and Hartley’s method: The principle of the method is illustrated in Figure. The apparatus consists of two concentric cylindrical tubes, A and B. The porous tube, A, carries in it the semi-permeable membrane of copper ferrocyanide. The inner tube is surrounded in the outer tube, B. The outer tube has an opening at C through which pressure could be applied from outside. The porous tube A has two side tubes, D and E. The solvent is added to A through E. The side tube is a capillary tulle so that any small change in pressure in A could be easily detected by the movement of the liquid meniscus in the tube.
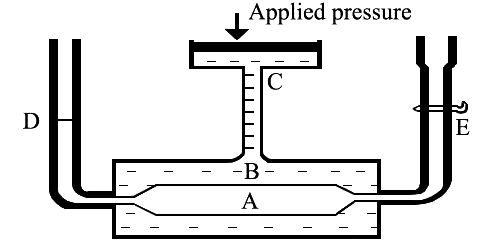
Fig: Berkeley and Hartley’s pressure apparatus
Measurement of Osmotic Pressure: Different methods are in use for the measurement of osmotic pressure in the laboratory but Berkley and Hartley’s method gives the preeminent results. The apparatus consists of an absorbent pot containing copper ferrocyanide deposited in its wall (acts as a semi-permeable membrane) and fixed into a bronze cylinder to which is fixed a piston and a pressure determine (to read the applied pressure).
Procedure – The porcelain tube is filled with pure solvent and the metallic jacket with a solution. The level in the capillary tube will tend to move down as the solvent flows towards solution due to osmosis. External pressure is now applied on the solution by the piston so that level in capillary remains stationary. The reading of pressure gauge is recorded. This is the osmotic pressure of the solution.
The pot is fixed with a tube indicator on left and water reservoir on right. Pot is filled with water while the cylinder is filled with a solution whose osmotic pressure is to be measured. Water tends to pass into the solution through the semipermeable membrane with the result that the water level in the indicator falls down. External pressure is now applied with the piston so as to preserve a constant level in the indicator. This external pressure is osmotic pressure.
Due to osmosis the water from tube A flows to the solution in B and causes a movement in the liquid meniscus in D. Berkeley and Hartley prevented the inflow of water on tube B by applying an external pressure through C and maintained the liquid meniscus in at the position until equilibrium. The excess pressure thus applied to the solution side to prevent water inflow gave the osmotic pressure at the temperature of the experiment. The apparatus is capable of giving good results in a relatively short time compared to other methods where the solvent is allowed to flow into the solution. In this method, there is no change in the concentration of the solution because no solvent is allowed to enter the solution tube. Pressure up to 150 atmospheres can be measured by this method. Some results of Berkeley and Hartley are given in Table.
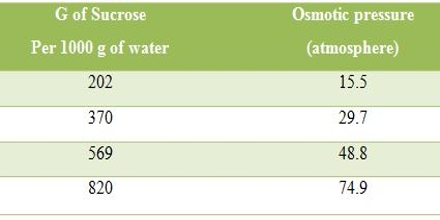
Osmotic pressure of Sucrose solutions at 300 C