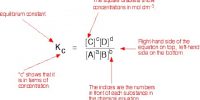
A chemical equilibrium actually involves two opposing reactions. One favoring the formation of products and the other favoring the formation of reactants. If the forward reaction in a chemical equilibrium is endothermic (accompanied by absorption of heat) then the reverse reaction is exothermic (accompanied by the evolution of heat).
Let consider the example
N2O4 (g) 2NO2 (g) ; ΔH = +59.0 kJ/mole
In this equilibrium, the reaction of the product formation (NO2) is endothermic in nature and therefore, the reverse reaction of reactant formation (N2O4) should be exothermic. If the above equilibrium reaction mixture is heated then its temperature will be raised. According to Le Chatelier’s principle, the equilibrium will shift in the direction which tends to undo the effect of heat. Therefore, the equilibrium will shift towards the formation of NO2 and subsequent dissociation of N2O4 increases. Therefore, generally, when the temperature is raised in a chemical equilibrium, among the forward and reverse reactions, the more endothermic reaction will be favored. Similarly, if the temperature of the equilibrium is decreased i.e., cooled, then the exothermic reaction among the forward and reverse reaction of the equilibrium will be favored.