Effect of change of pressure
If a system in equilibrium consists of reactants and products in gaseous state, then the concentrations of all components can be altered by changing the total pressure of the system. Consider the equilibrium in the gaseous state such as
N2O4 (g) ↔ 2NO2 (g)
Increase in the total pressure of the system in equilibrium will decrease the volume proportionately. According to Le Chatlier’s principle, the change can be counteracted by shifting the equilibrium towards decreasing the moles of products.
Hence, the reaction of combination of NO2 molecules to N2O4 formation will be favored.
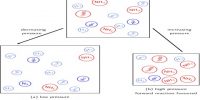
In case of a gas phase equilibrium which is accompanied by decrease in number of moles of products formed, the effect of pressure can be considered as follows,
N2 (g) + 3H2 (g) ↔ 2NH2 (g)
If the pressure is increased then the volume will decrease proportionately.
Consequently, the equilibrium will shift in the direction in which there is a decrease in the total number of moles, ie., favours the formation reaction of NH3. Here from four moles of reactants two moles of NH3 are formed. Thus at higher pressures, the yield of ammonia will be more.