Discovery of Protons and Neutrons
Electrical discharge carried out in the modified cathode ray tube led to the discovery of particles carrying positive charge, also known as canal rays. The characteristics of these positively charged particles are listed below.
(i) unlike cathode rays, the positively charged particles depend upon the nature of gas present in the cathode ray tube. These are simply the positively charged gaseous ions.
(ii) The charge to mass ratio of the particles is found to depend on the gas from which these originate.
(iii) Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
(iv) The behavior of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
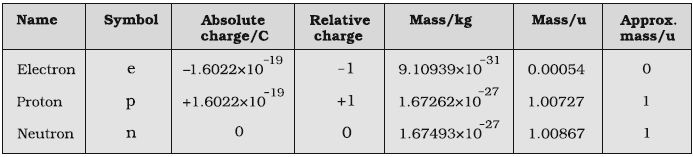
The smallest and lightest positive ion was obtained from hydrogen and was called proton. This positively charged particle was characterized in 1919. Later, a need was felt for the presence of electrically neutral particle as one of the constituent of atom. These particles were discovered by Chadwick (1932) by bombarding a thin sheet of beryllium by a-particles. When electrically neutral particles having a mass slightly greater than that of the protons was emitted. He named these particles as neutrons. The important properties of these fundamental particles are given in Table.