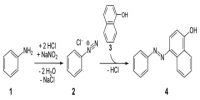
Markovnikov rule is quite general but not universal. In 1933, the American chemist M.S. kharach discovered that the addition of HBr to non-symmetrical alkenes, in the presence of organic peroxide (R — O — O — R), takes a course opposite to that suggested by Markovnikov i.e. the negative part of the reagent becomes attached to that double bonded carbon which contains maximum number of H atoms.
For example, when propene reacts with HBr in presence of peroxide, the major product is n-propyl bromide, whereas in the absence of peroxide, the major product is isopropyl bromide.
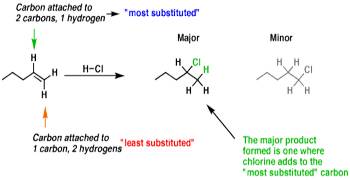
The reaction follows free radical mechanism.