Each ice crystal may be considered as a giant molecule. In ice, each oxygen atom is linked to four hydrogen atoms- two of them by covalent bonds and two by hydrogen bond. The covalently bonded hydrogen atoms are at a distance of 0.96A from the oxygen atom. While the hydrogen-bonded hydrogen atoms are at a distance of 1.80A from the oxygen atom. The arrangements of hydrogen atoms are distorted tetrahedral. In this way, a giant molecule is formed, so the real molecular formula of ice is (H2O)n. In this structure, there are many vacant spaces, which arc like vacant cages. For this reason, the density of ice is lower than that of water.
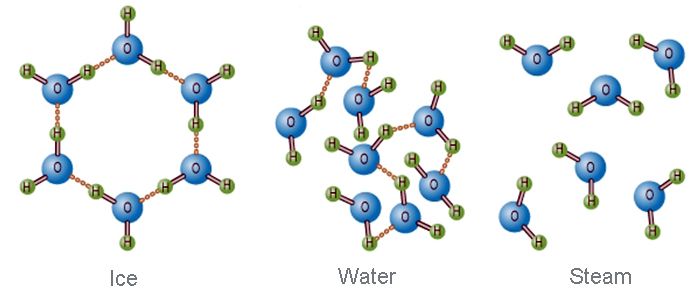
Fig: Molecular structure of Ice, Water, and Steam.












