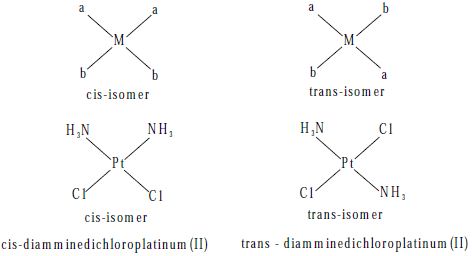
Geometric isomers are possible for both square planar and octahedral complexes, but not tetrahedral. In a cis-isomer two identical (or) similar groups are adjacent to each other whereas in a trans-isomer they are diametrically opposite to each other.
Square planar complexes of the type [Ma2b2]n± where a and b are monodentate ligands, exist as cis and trans-isomers as shown below.
Example of this type of complexes are [Pt (NH3)2 Cl2] and [Pd(NH3)2 (NO2)2]. The cistrans isomers of these compounds are represented as