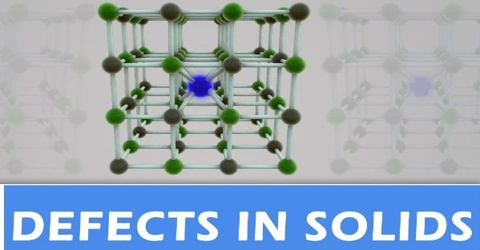
Defects in Solids
A crystal is considered to be ideal or perfect if there is regular arrangement of lattice points, and there is no irregularity of any kind in the crystal lattice. Real crystals, however, feature defects or irregularities in the arrangements of atoms, molecules or ions in the lattice points. Any deviation from a chemically pure, stoichiometric perfect crystal is called an imperfection or a defect.
However, depending on how these defects arise in crystal lattice, there are basically two types of defects:
(i) Intrinsic (also called thermodynamic defect) and
(ii) Extrinsic (also called non-equilibrium defect).
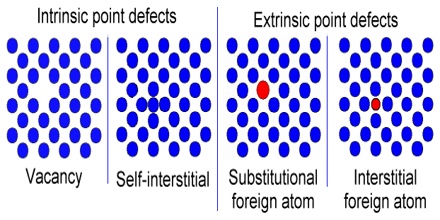
(i) Intrinsic defect: The intrinsic detects occur when certain configurationally disorder is introduced into the crystal lattice spontaneously at an elevated temperature. This is a direct consequence of the laws of thermodynamics.
(ii) Extrinsic defect: The extrinsic defects are deliberately introduced into the crystal lattice to manipulate the properties of a particular crystal system.