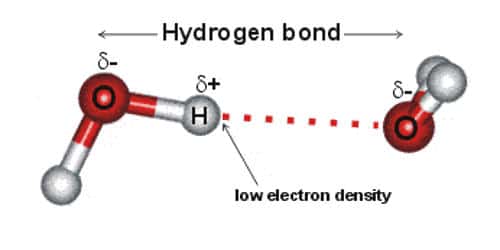
Hydrogen bonding comes into existence as a result of dipole-dipole interactions between the molecule in which hydrogen atom is covalently bonded to a highly electronegative atom. Hydrogen bonds are a strong type of dipole-dipole interaction.
In a molecule, when a hydrogen atom is linked to a highly electronegative atom, it attracts the shared pair of electrons more and so this end of the molecules becomes slightly negative while the other end becomes slightly positive. The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This bond is called the hydrogen bond.
There are two requirements for hydrogen bonding –
- First molecules have a hydrogen attached to a highly electronegative atom (N, O, F). (hydrogen bond donor)
- The second molecule has a lone pair of electrons on a small highly electronegative atom (N, O, F). (hydrogen bond acceptor)
Therefore, the conditions for effective hydrogen bonding are:
(i) high electronegativity of the atom bonded to hydrogen atom so that the bond is sufficiently polar. The molecule must contain a highly electronegative atom linked to the hydrogen atom. The higher the electronegativity more is the polarization of the molecule.
(ii) the small size of the atom bonded to hydrogen so that it is able to attract the bonding electron pair effectively. The size of the electronegative atom should be small. The smaller the size, the greater is the electrostatic attraction.
Effects of Hydrogen Bonding on Elements –
- Association
The molecules of carboxylic acids exist as dimers because of hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formula.
- Dissociation
In aqueous solution, HF dissociates and gives the difluoride ion instead of fluoride ion. This is due to hydrogen bonding in HF. The molecules of HCl, HBr, HI do not form a hydrogen bond. This explains the non-existence of compounds like KHCl2, KHBr2, KHI2.
If the atom bonded to hydrogen has a low value of electronegativity and/or large atomic size, dipole-dipole interactions are not strong enough to allow effective hydrogen bonding. As a result of hydrogen bonding, a hydrogen atom links the two electronegative atoms simultaneously, one by a covalent bond and the other by a hydrogen bond.
Only nitrogen, oxygen, and fluorine form strong hydrogen bonds because they have a high value of electronegativity and small atomic size. Hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; such a bond is weaker than an ionic bond or covalent bond but stronger than van der Waals forces.