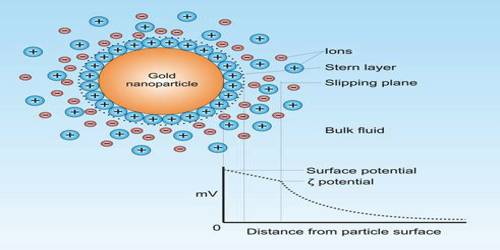
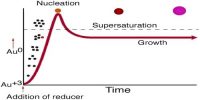
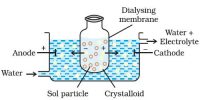
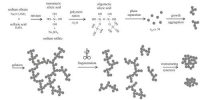
Color Property of Colloids
The color of a lyophobic colloid depends not only on the color of the disperse phase but also considerably on the particle size. The color of a colloidal solution depends upon the size of colloidal particles present in it. Larger particles absorb the light of longer wavelength and therefore transmit light of shorter wavelength. For example, a silver so having particles of size 150nm appears violet, whereas that having particles of size 60nm appears orange-yellow.
It is best known for its distribution of particles evenly throughout the solution without settling down. It is also sometimes called as colloidal dispersion. The best example to prove their familiarity with us is that we know from very early times that coagulation of milk results in the formation of curd.
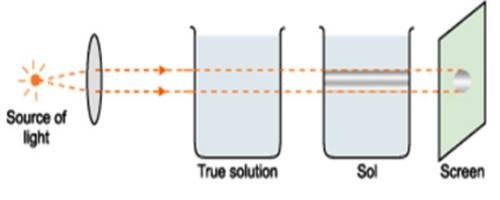
Properties of colloids and their variation are a well-known area ever since the primitive age. The same colloid system may appear to have different colors in white light depending on the size. A reasonable estimate of particle size can be made from the color of the sol in transmitted light and reflected light. Thus a sulphur sol may be bright yellow, light yellow, or even white depending on the size. The colour of a colloidal solution depends upon the size of colloidal particles present in it. Larger particles absorb the light of longer wavelength and therefore transmit light of shorter wavelength. Arsenious sulphide sol, which is normally bright yellow in transmitted and reflected light, may appear bluish yellow in reflected light depending on the particle size. The difference in the refractive index of the dispersion medium and disperse phase also greatly controls the color. For example, a silver so having particles of size 150nm appears violet, whereas that having particles of size 60nm appears orange-yellow.
Many sols are colored. Sol particles are able to scatter light rays. The Colour of the sol depends upon the wavelength of scattered light by the sol particles and which again depends on the size of the sol particles. The color of the colloidal solution also changes with the way the observer receives the light. Let us consider silver sol (colloidal solution of the same substance) having different types of particles. It is found that the sols show different colors.
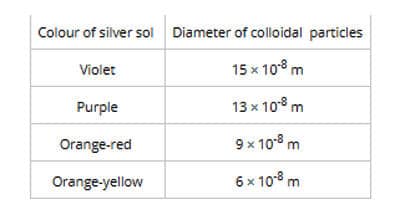
The size of the colloidal particles present in the solution determines the color of the colloidal dispersion. Larger the size of the particles longer will be the wavelengths of light being absorbed and hence these particles conduct small wavelengths.