Characteristics of an ideal gas:
The gases which follow fundamental postulates of the kinetic theory of gases and at all temperatures and pressures simultaneously obey both Boyle’s law and Charles’s law are called ideal gases.
- Gases consist of particles in constant, random motion. They continue in a straight line until they collide with something—usually each other or the walls of their container. Gas pressure is due to the molecules colliding with the walls of the container. No energy is lost or gained from collisions.
- An ideal gas obeys the equation PV = nRT at all temperatures and pressures. Ideal gases obtain no volume, unlike real gases which obtain small volumes.
- The internal energy of an ideal gas at constant temperature is not dependent on its That means, (du/dV)T = 0, here, u = internal energy of the gas, V = volume of the gas, T = temperature.
- No molecular forces are at work. There is no attraction or repulsion between the molecules of an ideal gas. This means that there is no attraction or repulsion between the particles.
- Particles are point masses with no volume. The total value of the gas molecules of an ideal gas is negligibly small compared with the value occupied by the gas molecules. The particles are so little compared to the space between them, that we do not consider their size in ideal gases.
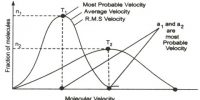
- The kinetic energy of a gas is a measure of its Kelvin temperature. Individual gas molecules have different speeds, but the temperature and kinetic energy of the gas refers to the average of these speeds.
- All gases at a given temperature have the same average kinetic energy. The average kinetic energy of a gas particle is directly proportional to the temperature. An increase in temperature increases the speed at which the gas molecules move.
The properties of an ideal gas are:
- An ideal gas consists of a large number of identical molecules. An ideal gas is different from a real gas in many ways.
- The volume occupied by the molecules themselves is negligible compared to the volume occupied by the gas.
- The molecules obey Newton’s laws of motion, and they move in random motion.
- The molecules experience forces only during collisions; any collisions are completely elastic and take a negligible amount of time.
- All gases at a given temperature have the same average kinetic energy.
- Lighter gas molecules move faster than heavier molecules.
- An ideal gas mass can be disregarded in the equation because it has none; this is because an ideal gas is said to be a particle and particles do not have any mass.
Ideal gases don’t experience any intermolecular forces –
- All of these answers are properties of ideal gases
- The gas particles have elastic collisions
- The gas particles have almost no mass
- The gas particles have no volume
- The gas particles have strong intermolecular forces acting on them.