ChemiCalculation of solubility product from solubility data
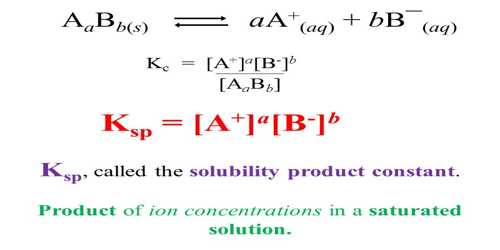
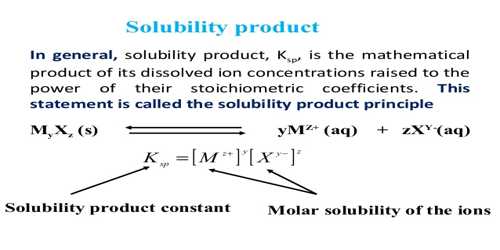
Solubility is defined as the maximum amount of solute that can be dissolved in a solvent at equilibrium. The solubility product constant (K) describes the equilibrium between a solid and its constituent ions in a solution. The solubility product of a compound can be calculated from solubility data. This will be explained with the help of few worked out examples.
Example- 1: The solubility of silver chloride, AgCl, is 1.92 x 10-3 g L-1 at 25°C. Calculate the solubility product of AgCl at this temperature.
Solution:
In solution AgCl (s) ↔ Ag+ (aq) + Cl– (aq)
Solubility in mol L-1 = [1.92 x 10-3 / 143.5] = 1.34 x 10-5
(Relative formula mass of AgCl = 143.51)
The concentration of each of the ions is equal to the solubility of the salt.
[Ag+] = [Cl–] = 1.34 x 10-5
Ksp = [Ag+] = [Cl–] = (1.34 x 10-5)2
= 1.8 x 10-10
Example- 2: At 250C the solubility of lead iodide, PbI2, is found to be 1.2 x 10-3 mol L-1. Calculate the solubility product, Ksp of lead iodide.
Solution
In solution, PbI2 (s) ↔ Pb2+ (aq) + 2I (aq).
The concentration, i.e. solubility, of the salt is equal to the concentration of Pb2+ ions. It can be seen that the concentration of iodide ion, I–, is double that of lead ion, Pb2+.
[Pb2+] = 1.2 x 10-3 mol L-1.
[I–] = 2 [Pb2+] = 2 x 1.2 x 10-3
= 2.4 x 10-3 mol L-1
Ksp = [Pb2+] [I–]2 = (1.2 x 10-3) x (2.4 x 10-3)2
= 6.9 x 10-9.