Boiling point of Water
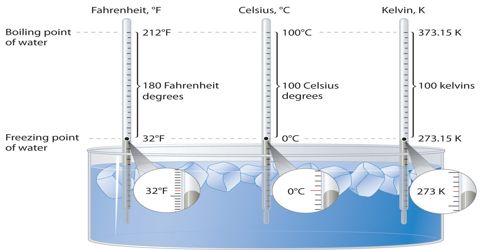
The boiling point of water is 100°C or 212° F at 1 atmosphere of pressure (sea level). When you take some water in a beaker and continue to heat it, what happens? The temperature of water increases and at certain temperature it begins to boil. The temperature at which water begins to vaporize is called the boiling point of water. Like water ach Bawd has its specific boiling point. Let us determine the boiling point of water.
Task: To determine the boiling point of water.
Required Accessories: One beaker, water, thermometer, spirit lamp etc.
Procedure: Take a beaker with half of it filled with water. Put the beaker on the spirit lamp. Immerse the thermometer in the water in the beaker as shown in the figure. Now apply heat and observe the temperature in the thermometer. When the temperature of the thermometer rises to 95 degree Celsius, carefully observe the condition of the modem of water in the beaker.
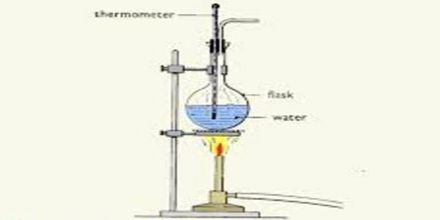
When the water begins to boil notice the temperature in the thermometer. This temperature is the boiling point of water. What is the reading of this temperature? It is 100 degree Celsius. The boiling point of water also depends on the purity of the water. Water which contains impurities (such as salted water) boils at a higher temperature than pure water. You can determine the boiling point of ether or spirit. But as the organic substances are combustible, you can not apply heat directly to them. You need to taking an aluminum pot with water and heat the beaker with ether or spirit in it.