Applications of Solubility Product Principle
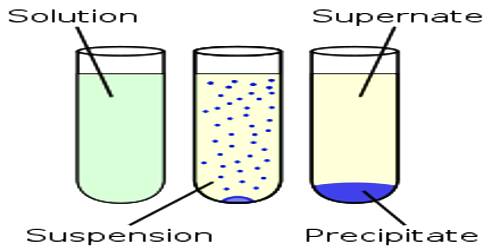
Qualitative analysis is mainly based on precipitating out the metal ions from a solution by the addition of a suitable reagent. A salt will be precipitated if the conditions are created such that QC values exceed their solubility products. For example, metal ions in Group II of the general analytical Table are precipitated as sulphide by passing H2S gas through the acidic solution of their salts.
As the solubility products of the sulphides of these metals are very low their sulphides are precipitated most easily, whereas those of the other metals remain in solution under the condition created. Solubility-product constants can be used to devise methods for separating ions in a solution by selective precipitation. Selective precipitation is used to form a solid with one of the ions in solution without disturbing the other ions. The solution is treated with various reagents to test for reactions characteristic of certain ions, which may cause the color change, solid forming, and other visible changes.
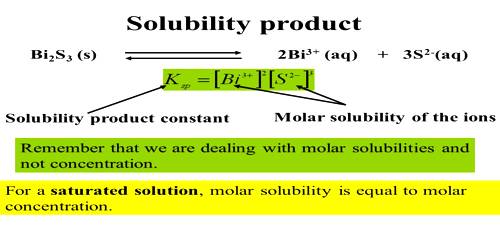
Example: What will be the maximum concentration of Cd2+ ions and Mn2+ ions that will remain in solution after precipitation by excess H2S in 0.25 mol L-1 HCl?
Solubility products of CdS and MnS are 1.4 x 10-28 and 1.4 x 10-15 respectively and [S2-] in the solution is 1.7 x 10-22 mol L-1.
Solution
In a solution containing S2- of concentration 1.7 x 10-22 mol L-1.
[Cd2+] = (1.4 x 10-28) / (1.3 x 10-8); as [Cd2+] [S2-] = (1.4 x 10-28)
= 1.1 x 10-20 mol L-1.
While, [Mn2+] = (1.4 x 10-15) / (1.3 x 10-8); as [Mn2+][S2-] = (1.4 x 10-15)
= 1.1 x 10-7 mol L-1.
It may be seen that the concentration of Cd2+ is practically nil compared to the concentration of Mn2+ ions, i.e., Cd2+ will he almost completely precipitated out on passing H2S in acidic solution while Mn2+ will remain in solution.