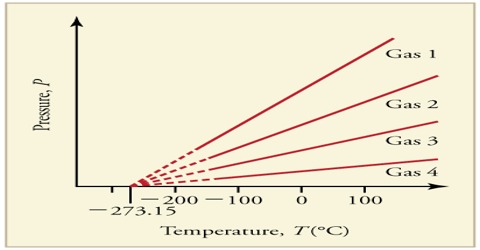
The Absolute Zero of Temperature
At – 273.16°C or 0°K the volume of any gas would theoretically be zero. In reality most gases become liquid long the absolute zero is reached. A temperature as low as absolute zero has never been reached but low temperature, scientists have been able to reach within a temperature of about 10-5 K. As the temperature of a substance is lowered the energy of its molecules also decreases. Consequently the movements of the molecules become less vigorous. At the absolute zero all movements of the molecules will cease or will be minimum. It has been shown theoretically by quantum mechanics that the only type of energy that the molecules may possess at O0 K is the energy due to vibrational motion and this energy at 0°K is known as the zero-point energy.
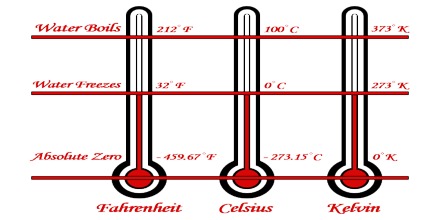
Although Figure shows that at 00 K the volume of a gas is zero, in reality it is not so. At this temperature the molecules would be compressed so close to each other that they would not have space to move. This volume, however, would be very small compared to the volume occupied by the substance in the gaseous state under ordinary temperatures.