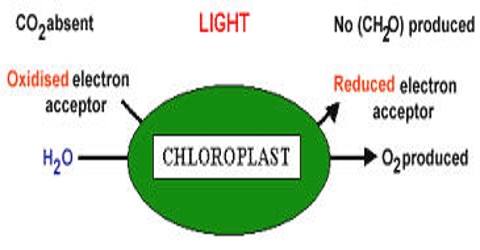
The Hill reaction is formally defined as the photoreduction of an electron acceptor by the hydrogens of water, with the evolution of oxygen. The source of oxygen which is evolved during the photosynthesis is water. In 1937, an English biochemist named, Robin Hill proved this idea by a reaction. He demonstrated that the process by which plants produce oxygen is separate from the process that converts carbon dioxide into sugars. This reaction is named as Hill reaction.
The Hill reaction is properly defined as the photoreduction of an electron acceptor by the hydrogen of water, with the advancement of oxygen. It is the light-dependent transfer of electrons by chloroplasts in photosynthesis that results in the cleavage of water molecules and the liberation of oxygen. In the organism the last electron acceptor is NADP+. We can measure the rate of the Hill reaction in isolated chloroplasts.
The reaction is as follows:
Robin hill put chloroplast water and some inorganic oxidizers; e.i., hydrogen acceptors together in absence of Carbon dioxide (CO2) and in presence of light is seen that no carbohydrate is produced in absence of carbon dioxide oxygen is released in this process. It is the light-driven transfer of electrons from water to Hill reagents (non-physiological oxidants) in a direction against the chemical potential gradient as part of photosynthesis. In fact, hydrogen of water releases oxygen by the reduction of a hydrogen acceptor. It is proved from Hill’s experiment that the oxygen evolved in photosynthesis comes out of water.
Inorganic oxidizers or Hydrogen acceptors + 2H2O → (light and Chlorophyll) → 2AH2 + O2
Light is absorbed and the energy is used to drive electrons from water to generate NADPH and to drive protons across a membrane. These protons return through ATP synthase to make ATP.
The Hill Reaction depends on electrons released during the light-dependent stage of photosynthesis being picked up by the blue electron acceptor DCPIP. The light-dependent reactions use light energy to make two molecules needed for the next stage of photosynthesis: the energy storage molecule ATP and the reduced electron carrier NADPH. The reaction can only occur if the thylakoid membranes are illuminated as the light-dependent stage stops in the dark (tube B in the procedure). In plants, the light reactions take place in the thylakoid membranes of organelles called chloroplasts.