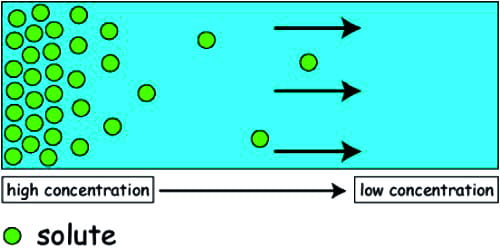
The potential ability of a substance to move from a region of high concentration to a region of low concentration at a constant, temperature end atmospheric pressure is called diffusion pressure. The diffusion pressure of the gas is a gas balloon that is higher than the diffusion pressure of its surrounding air. It is proportional to the concentration of diffusing particles. So when the balloon bursts, the gas inside the balloon spread throughout the surrounding air due to its higher diffusion pressure. As a result, if the balloon bursts, the air inside the balloon spreads all through the contiguous air due to its privileged diffusion pressure.
It is a hypothetical term coined by Meyer (1938) to denote the possible aptitude of the molecules or ions of any material to disseminate from an area of their higher concentration to that of their lower concentration.
Factors Affecting Diffusion: Diffusion of a substance depends on temperature, atmospheric pressure. The concentration of molecules of that substance and concentration of the medium.
- Temperature:
With the increase of temperature diffusion usually increases. The diffusion of a material depends on temperature, atmospheric pressure, absorption of molecules of that material and deliberation of the medium.
- The concentration of the substrate:
The rate of diffusion will be higher if the concentration of molecules of the substrate is higher; if the concentration is less, the rate of diffusion will be less. DP is directly proportional to the diffusing particles.
- The concentration of the medium:
If the concentration of the medium, like water or air, is more, the rate of diffusion will be less; and if the concentration is less the rate of diffusion will be high. DP of pure water is maximum.
- Atmospheric pressure:
The increase in atmospheric pressure will reduce the rate of diffusion and a decrease in atmospheric pressure will increase the rate. Usually, temperature and pressure at a certain place remain the same at a certain time, in that case, the concentration of the substance and the concentration of the medium become the factors to control diffusion. If the medium and the diffusion substance are the same (as in the balloon and the surrounding air) then diffusion will continue until the concentration of the two becomes the same.
Importance of Diffusion:
(i) Diffusion keeps the cell walls of the interior plant tissues humid.
(ii) It is a means of spreading of ions and other substances throughout the protoplast.
(iii) Transpiration or loss of water in vapor forms is a diffusion procedure.
(iv) Exchange of gases between the plant interior and outside air occurs through diffusion.